原电池反应通常是放热反应,在理论上可设计成原电池的化学反应是
| A.C(s)+H2O(g)= CO(g)+H2(g) |
| B.HCl + NaOH = NaCl + H2O |
C.Ca(OH)2+2NH4Cl 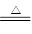 CaCl2 + 2NH3↑ + 2H2O CaCl2 + 2NH3↑ + 2H2O |
| D.CH4(g)+2O2(g)= CO2(g)+2H2O(l) |
原电池反应通常是放热反应,在理论上可设计成原电池的化学反应是
| A.C(s)+H2O(g)= CO(g)+H2(g) |
| B.HCl + NaOH = NaCl + H2O |
C.Ca(OH)2+2NH4Cl 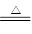 CaCl2 + 2NH3↑ + 2H2O CaCl2 + 2NH3↑ + 2H2O |
| D.CH4(g)+2O2(g)= CO2(g)+2H2O(l) |
试题篮
()