已知:Fe(s)+ O2(g)==="FeO(s)" ΔH1=-272 kJ/mol
O2(g)==="FeO(s)" ΔH1=-272 kJ/mol
2Al(s)+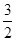 O2(g)===Al2O3(s)ΔH2=-1 675 kJ/mol
O2(g)===Al2O3(s)ΔH2=-1 675 kJ/mol
则2Al(s)+3FeO(s)===Al2O3(s)+3Fe(s)的ΔH是
| A.859 kJ/mol | B.-859 kJ/mol |
| C.-1403 kJ/mol | D.-2491 kJ/mol |
已知:Fe(s)+ O2(g)==="FeO(s)" ΔH1=-272 kJ/mol
O2(g)==="FeO(s)" ΔH1=-272 kJ/mol
2Al(s)+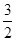 O2(g)===Al2O3(s)ΔH2=-1 675 kJ/mol
O2(g)===Al2O3(s)ΔH2=-1 675 kJ/mol
则2Al(s)+3FeO(s)===Al2O3(s)+3Fe(s)的ΔH是
| A.859 kJ/mol | B.-859 kJ/mol |
| C.-1403 kJ/mol | D.-2491 kJ/mol |
试题篮
()