已知lg肼(N2H4)气体燃烧生成氮气和水蒸气,放出16.7kJ的热量。下列热化学方程式
书写正确的是
A.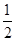 N2H4(g)+ N2H4(g)+ 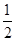 O2(g)= O2(g)= 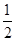 N2(g)+H2Og)△H=-267.2kJ·mol-l N2(g)+H2Og)△H=-267.2kJ·mol-l |
| B.N2H4+O2=N2+2H2O△H=-534.4kJ·mol-l |
| C.N2H4(g)+O2(g)=N2(g)+2H2O(1)△H=-534.4kJ·mol-l |
| D.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H=+534.4kJ·mol-l |
已知lg肼(N2H4)气体燃烧生成氮气和水蒸气,放出16.7kJ的热量。下列热化学方程式
书写正确的是
A.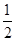 N2H4(g)+ N2H4(g)+ 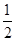 O2(g)= O2(g)= 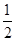 N2(g)+H2Og)△H=-267.2kJ·mol-l N2(g)+H2Og)△H=-267.2kJ·mol-l |
| B.N2H4+O2=N2+2H2O△H=-534.4kJ·mol-l |
| C.N2H4(g)+O2(g)=N2(g)+2H2O(1)△H=-534.4kJ·mol-l |
| D.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H=+534.4kJ·mol-l |
试题篮
()